Chicken, Egg or Both?
Are many or even most of the health challenges in modern Western societies—high blood pressure, high blood sugar, high cholesterol levels, cardiovascular disease in general, weight problems, etc.—related? There is a good case to be made that they are.
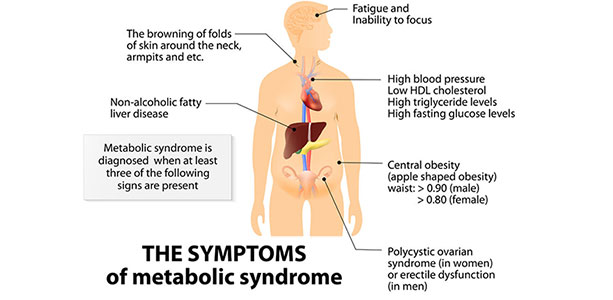
At the core of Syndrome X, now much more commonly known as the metabolic syndrome, are dysregulations and dysfunctions involving glucose and insulin. These manifest as central obesity (excessive fat around the belly), high blood pressure and blood fat disorders—especially hypertriglyceridemia and low levels of HDL cholesterol. In addition to participating in the development of many facets of the metabolic syndrome, impairment in insulin sensitivity also appears to be involved in the aging process by promoting inflammation, endothelial dysfunction (problems with the blood vessels), the production of advanced glycation end products (AGE) and oxidative stress. Downstream consequences of these dysfunctions include cardiovascular disease and cancer. These issues were discussed approximately one year ago in this magazine under the heading “Is the Metabolic Syndrome a Consequence of Aging?” (May 2017) Nonalcoholic fatty liver disease was not originally included in metabolic syndrome manifestations. However, over the past decade medical thinking by almost all parties regarding the relationship of these conditions has moved strongly towards viewing them as linked in some way. From 15 to 33 percent of the worldwide population is estimated to suffer from non-alcoholic fatty liver disease.
As noted in the earlier article, major questions remain regarding the early appearance of the metabolic syndrome. Western medicine, unlike, for instance, traditional Chinese and Indian medicine, tends to pursue and treat the various arms of the metabolic syndrome as distinct clinical entities. Much of the research on the syndrome within allopathic medicine over the past three decades has been aimed at arguing against this separation. Questions regarding the liver and metabolic syndrome involve similar issues. According to some authorities, non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of the metabolic syndrome. However, others believe NAFLD is a distinct entity that actually initiates the metabolic syndrome. The choice as to which comes first, the metabolic syndrome or NAFLD, if indeed they are distinct, helps to determine how each can be prevented and treated.
Early Signs of the Metabolic Syndrome
Most clinicians consider a level of circulating glucose under fasting conditions in the range of 100–125 mg/dl to be prediabetic, but agreement beyond this is hard to come by. Normal levels of blood glucose and circulating insulin, meaning levels that do not lead to components of the metabolic syndrome, remain in dispute. Nevertheless, evidence suggests that surprisingly “normal” fasting blood glucose levels still have consequences.
Circulating glucose within levels generally accepted as normal can influence brain function in an unfavorable manner. Similarly, increasing hemoglobin A1C (HbAlC) and insulin levels even in the non-diabetic range can affect blood pressure adversely. Such observations lead to suspicions that minor insulin resistance predicts the early onset of many disturbed health parameters involved in the metabolic syndrome. Recently, Harry G. Preuss and co-authors, of whom I am one, addressed just such questions in “General Lack of Correlations between Age and Signs of the Metabolic Syndrome in Subjects with Non-diabetic Fasting Glucose Values.”1
Based on our study, the health data were significantly better with fasting glucose levels in the range of 67–86 mg/dL than at 98–125 mg/dL. Across a wide range of variables lower definitely was better leading to the conclusion that decreasing insulin resistance and maintaining fasting glucose levels at the low end of the normal range is highly desirable. Advancing years did not appear to be a factor leading to the metabolic syndrome. In terms of the present topic, using the data at hand, advancing age per se also did not seem to affect liver health.
Non-Alcoholic Fatty Liver Disease and the Metabolic Syndrome in Non-Diabetics
If aging is not a primary factor in the appearance of the metabolic syndrome, yet NAFLD and the metabolic syndrome are linked, some other cause or causes are at work. The usual list of suspects can be found in the typical elements examined to determine diagnosis.
Aside from ruling out an elevated consumption of alcohol, a verdict of NAFLD primarily is based on discovering excess liver fat accumulation along with raised circulating aminotransferase levels generally involving ALT more than AST. For clarity, the latter are “two important transaminase enzymes…aspartate transaminase (AST), also known as serum glutamic oxaloacetic transaminase (SGOT); and alanine transaminase (ALT), also called alanine aminotransferase (ALAT) or serum glutamatepyruvate transaminase (SGPT)… [Transaminases] are important in the synthesis of amino acids, which form proteins.”2 Liver health and function is evaluated by, among other markers, the levels of ALT, ALS and their ratio. Whenever a patient gets blood drawn for basic blood panel tests, ALT and ALS are checked.
Alanine aminotransferase (ALT) found persistently to be elevated often is viewed as indicating the presence of NAFLD. More generally, an elevated circulating concentration of ALT is recognized to be a fairly specific sign for liver injury. In considering the “normal” range of ALT (<40 U/L) for cardiovascular disease in general, unfortunately the normal range does not represent a cut-off below which one is safe, but, instead, a point of a continuum of risk.3
Starting with a subject population that is non-diabetic and without any of the normal signs of liver dysfunction, the question, again, is whether NAFLD is a component of the metabolic syndrome or, instead, is an independent disorder that precedes and actually initiates the onset of the syndrome. The answer to this question has important implications for the treatment of both conditions. On the one hand, if the metabolic syndrome, meaning primarily insulin resistance, is the central issue and NAFLD is really just a component of the syndrome, even should there be feedback once NAFLD is established, then the metabolic syndrome is tackled first to prevent or treat its manifestations in the liver. On the other hand, if the hepatic condition is first in time and in causality, treatment starts with the liver.
Treating NAFLD: The Liver Or Insulin Resistance
A major reason for looking closely at the relationship between the metabolic syndrome and NAFLD is that treating the latter with approaches aimed specifically at the liver has yielded underwhelming levels of success. The single most successful standalone natural ingredient probably is mixed tocotrienols taken 200 mg twice per day for a year.4 Overall, 13 of 26 subjects (50 percent) became NAFLD-negative. In those with a mild form, there was a 38 percent decrease in the number of subjects with active disease at the end of the study, indicating that improvement was linked to the severity of the condition. Somewhat less impressive was silybin (an active component of milk thistle extract) combined with phosphatidylcholine and vitamin E acetate (á-tocopherol), again, taken for twelve months. Still, the formula significantly improved liver conditions associated with NAFLD (steatosis, lobular inflammation, ballooning, and fibrosis) and the overall NAFLD Activity Score.5 Interestingly, in another trial the results with milk thistle and vitamin E were much improved in patients who followed a low calorie diet with weight loss.6
Findings of this sort strengthen the argument that NAFLD is an aspect of the metabolic syndrome and not its cause. A number of studies have demonstrated that hepatic insulin resistance likely is the chief culprit.7 For instance, the accumulation of triglycerides in the liver is a cause of the condition. Technically, hyperinsulinemia promotes the upregulation of genes that promote de novo lipogenesis (biosynthesis of fat) in the liver.8,9
Given evidence that de novo lipogenesis, which primarily is induced by high insulin levels and refined carbohydrates, is an important agent in the development of liver dysfunction, it can come as no surprise that an inhibitor of this process, (–)-hydroxycitric acid (HCA), improves liver function in experimentally-induced nonalcoholic steatohepatitis (NASH), a component of NAFLD. In animal experiments, both liver fibrosis and markers of liver function were improved under experimental conditions with ingestion of HCA.10,11 It should be noted that, experimentally, liver dysfunction is closely associated with the intake of rapidly absorbed carbohydrates, but not necessarily carbohydrate consumption itself.12 In humans, short-term fructose consumption in either isocaloric exchange or in hypercaloric supplementation promotes the development of hepatic insulin resistance in non-diabetic adults without affecting peripheral or muscle insulin sensitivity.13 Indeed, at this point there is little doubt but that fructose consumption in excess is a primary driver of the synthesis of triglycerides and other perturbations of the handling of fats by the liver leading to fatty liver disease.14 Fructose appears to induce both NAFLD and metabolic syndrome through related mechanisms.15 A steadily growing body of evidence supports the position that medium and long-term excess ingestion of fructose and rapidly absorbed carbohydrates progressively distort the handling of glucose and insulin not just in the liver, but also in the muscles and other lean tissues.
Back to the Chicken and Egg Question
So, what do we have so far? Evidence from animal models and human experience indicates that certainly one or more of the drivers of liver dysfunction—fructose and rapidly absorbed carbohydrates—act initially on the liver to cause insulin resistance. Liver insulin resistance subsequently makes general control of blood sugar levels more difficult and causes the body to release more insulin and/or insulin in greater amounts at a given time to regulate blood sugar, an action with particularly unfortunate consequences after meals. Over time, elevated glucose and insulin levels originally caused by the liver lead to insulin resistance in the muscles and other lean tissues, tissues that account for approximately 70 percent of all glucose clearance in the body.
In addition, there is a feedback loop between the liver and peripheral lean tissues that involves the metabolism of fats for energy and storage. Initially, circulating elevated levels of blood glucose result from hepatic insulin resistance whereas increased circulating free fatty acid concentrations are a primary expression of peripheral insulin resistance.16 Insulin, of course, controls the clearance and energy metabolism of both fuel sources; as a storage hormone, insulin in excess directly impedes the use of fats for energy, hence contributes to elevations in blood free fatty acids and triglycerides. The effects are bidirectional: insulin resistance leads to fat accumulation and fat accumulation amplifies insulin resistance.
This line of argument and supporting evidence suggest that the metabolic syndrome and NAFLD generally are linked from the start and take form largely concurrently. Forthcoming work by the team of Harry Preuss et al, indicates exactly this. ALT and the ratio of AST/ALT correlate significantly with fasting blood glucose in non-diabetics indicating that these markers of liver health move largely in unison with changes in insulin sensitivity. Insulin resistance drives both the metabolic syndrome and NAFLD.
What Is To Be Done?
Key to improving liver health are diet and exercise habits. This fact showed up in the treatment option mentioned above which combined milk thistle with vitamin E—results were much better in the subjects on a low calorie diet. Nutrients such as tocotrienols, milk thistle, quercetin and other liver supports and detoxifiers only marginally address the primary issues. These primary issues include:
- Consumption of rapidly absorbed carbohydrate sources, especially fructose, as discussed in the foregoing text
- Ingestion of foods that lead to elevated insulin release when eaten with refined carbohydrates; these include red meat and branched-chain amino acid sources17
- Reduced metabolism of fats for energy; certain food combinations, such as fats eaten with rapidly absorbed carbohydrates, severely interfere with the utilization of consumed fats for energy with negative implications for both liver and muscle insulin sensitivity
- Reliance on ultra-processed foods, meaning a preponderance of the foods now eaten in the US and many European nations 18,19,20 (see “Calories Don’t Add Up“
- A lack of regular exercise: 15–30 minutes twice per day is recommended
For success, the right tool must be applied for the proper purpose. Although it is true that the environment increasingly is polluted with various toxins, NAFLD predominantly is a result of the foregoing issues. Correspondingly, corrective nutrients and herbs for NAFLD, just as with the metabolic syndrome, are those that improve insulin sensitivity, fatty acid metabolism and metabolic fitness.
It also is useful to consider here once again observations made in “Nutrient Combining”
- Low glycemic index diets improve glycemic (blood sugar) response and variability as well as promote the metabolism of fat for energy; they may promote longterm health.21,22
- Taken in a milkshake, fructose (30 g) increased postprandial lipemia by 37 percent compared with control; glucose (17.5 g) increased postprandial lipemia by 59 percent.23 (Lipemia is the presence in the blood of an abnormally high concentration of emulsified fat, meaning primarily triglycerides, not cholesterol.)
- In Syndrome X/insulin resistant subjects (BMI of 30), glucose consumption (50 g) led to a 15.9 percent greater glycemic response and a 30.9 percent greater insulin response than did fructose (50 g). This is true in part because fructose is processed in the liver and then released later as glucose and/or converted into fat.
- On an energy balanced diet in these same subjects, fructose compared with glucose increased carbohydrate oxidation 31 percent, but decreased fat oxidation by 39 percent.24
- Low-fat/high-carbohydrate diets in Syndrome X individuals reduce levels of HDL cholesterol and increase triacylglycerol concentrations.25
- Sucrose is glucose + fructose; lactose is glucose + galactose; grape sugar (dextrose) is glucose.
Conclusion
There is an old Chinese medical observation to the effect, “disease enters the body by way of the mouth.” Both the metabolic syndrome and non-alcoholic liver disease would appear to offer evidence for the correctness of this judgment. They also would appear to buttress another ancient opinion, this time from the ancient Greek physician Galen, who considered many of the conditions that today we associate as cardiovascular as arising from the liver and only subsequently manifesting in the heart, the circulation, and so forth. Liver health, as evidenced by subtle changes in certain markers, is closely linked to insulin sensitivity and energy metabolism. As such, the best approach to liver issues that are not related to the ingestion of toxins per se is to be found in diet and exercise habits.
Endnotes
- Preuss HG, Mrvichin N, Clouatre D, Bagchi D, Preuss JM, Perricone NV, Swaroop A, Kaats GR. General Lack of Correlations between Age and Signs of the Metabolic Syndrome in Subjects with Non-diabetic Fasting Glucose Values. J Am Coll Nutr. 2017 Sep–Oct;36(7):556–64.
- https://en.wikipedia.org/wiki/Transaminase
- Porter SA, Pedley A, Massaro JM, Vasan RS, Hoffmann U, Fox CS. Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013 Jan;33(1):139–46.
- Magosso E, Ansari MA, Gopalan Y, Shuaib IL, Wong JW, Khan NA, Abu Bakar MR, Ng BH, Yuen KH. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo-controlled clinical trial. Nutr J. 2013 Dec 27;12(1):166.
- Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, Chiaramonte M, Cursaro C, Danila M, de Sio I, Floreani A, Freni MA, Grieco A, Groppo M, Lazzari R, Lobello S, Lorefice E, Margotti M, Miele L, Milani S, Okolicsanyi L, Palasciano G, Portincasa P, Saltarelli P, Smedile A, Somalvico F, Spadaro A, Sporea I, Sorrentino P, Vecchione R, Tuccillo C, Del Vecchio Blanco C, Federico A. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med. 2012 May 1;52(9):1658–65.
- Aller R, Izaola O, Gómez S, Tafur C, González G, Berroa E, Mora N, González JM, de Luis DA. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci. 2015 Aug;19(16):3118–24.
- Sesti G, Fiorentino TV, Hribal ML, Sciacqua A, Perticone F. Association of hepatic insulin resistance indexes to nonalcoholic fatty liver disease and related biomarkers. Nutr Metab Cardiovasc Dis. 2013 Dec;23(12):1182–7.
- Kawano Y, Cohen DE. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J Gastroenterol. 2013 Apr;48(4):434–41.
- Geisler CE, Renquist BJ. Hepatic lipid accumulation: cause and consequence of dysregulated glucoregulatory hormones. J Endocrinol. 2017 Jul;234(1):R1–R21.
- Surapaneni KM, Vishnu Priya V, Mallika J. Effect of pioglitazone, quercetin, and hydroxy citric acid on vascular endothelial growth factor messenger RNA (VEGF mRNA) expression in experimentally induced nonalcoholic steatohepatitis (NASH). Turk J Med Sci. 2015;45(3):542–6.
- Surapaneni KM, Jainu M. Pioglitazone, quercetin and hydroxy citric acid effect on hepatic biomarkers in Non Alcoholic Steatohepatitis. Pharmacognosy Res. 2014 Apr;6(2):153–62.
- Scribner KB, Pawlak DB, Ludwig DS. Hepatic steatosis and increased adiposity in mice consuming rapidly vs. slowly absorbed carbohydrate. Obesity (Silver Spring). 2007 Sep;15(9):2190–9.
- Ter Horst KW, Schene MR, Holman R, Romijn JA, Serlie MJ. Effect of fructose consumption on insulin sensitivity in nondiabetic subjects: a systematic review and meta-analysis of diet-intervention trials. Am J Clin Nutr. 2016 Dec;104(6):1562–76.
- Softic S, Cohen DE, Kahn CR. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig Dis Sci. 2016 May;61(5):1282–93.
- Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010 May;7(5):251–64.
- Lonardo A, Ballestri S, Marchesini G, Angulo P, Loria P. Nonalcoholic fatty liver disease: a precursor of the metabolic syndrome. Dig Liver Dis. 2015 Mar;47(3):181–90.
- Bremer AA, Mietus-Snyder M, Lustig RH. Toward a unifying hypothesis of metabolic syndrome. Pediatrics. 2012 Mar;129(3):557–70.
- Martínez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, Monteiro CA. Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ Open. 2016 Mar 9;6(3):e009892.
- Monteiro CA, Moubarac JC, Levy RB, Canella DS, Louzada MLDC, Cannon G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2017 Jul 17:1–9.
- Moreira PV, Baraldi LG, Moubarac JC, Monteiro CA, Newton A, Capewell S, O’Flaherty M. Comparing different policy scenarios to reduce the consumption of ultra-processed foods in UK: impact on cardiovascular disease mortality using a modelling approach. PLoS One. 2015 Feb 13;10(2):e0118353.
- Henry CJ, Kaur B, Quek RYC, Camps SG. A Low Glycaemic Index Diet Incorporating Isomaltulose Is Associated with Lower Glycaemic Response and Variability, and Promotes Fat Oxidation in Asians. Nutrients. 2017 May 9;9(5).
- Bennett CB, Chilibeck PD, Barss T, Vatanparast H, Vandenberg A, Zello GA. Metabolism and performance during extended high-intensity intermittent exercise after consumption of low- and high-glycaemic index pre-exercise meals. Br J Nutr. 2012 Aug;108 Suppl 1:S8–90.
- Singleton MJ, Heiser C, Jamesen K, Mattes RD. Sweetener augmentation of serum triacylglycerol during a fat challenge test in humans. J Am Coll Nutr 1999 Apr;18(2):179–85.
- Tittelbach TJ, Mattes RD, Gretebeck RJ. Post-exercise substrate utilization after a high glucose vs. high fructose meal during negative energy balance in the obese. Obes Res 2000 Oct;8(7):496–505.
- Poppitt SD, Keogh GF, Prentice AM, Williams DE, Sonnemans HM, Valk EE, Robinson E, Wareham NJ. Long-term effects of ad libitum low-fat, high-carbohydrate diets on body weight and serum lipids in overweight subjects with metabolic syndrome. Am J Clin Nutr. 2002 Jan;75(1):11–20.